

产品分类
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
联系我们
bionet生物反应器、coolvacuum冻干机、fiocchetti制冰机
 您现在的位置:斯沃德(北京)仪器设备有限公司 > 产品展示
您现在的位置:斯沃德(北京)仪器设备有限公司 > 产品展示 | 当前位置:产品展示 >> 冻干袋 >> lyoprotect冻干袋cGMP |
| lyoprotect冻干袋cGMP [返回] |
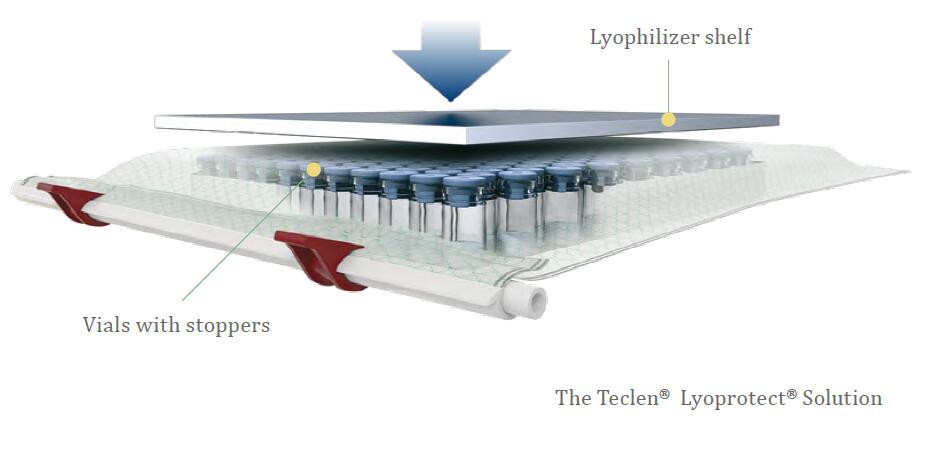 |
If aseptic criteria must be met, filling of vials, placement and closure of the bag
|
 客慕coolvacuum 冻干机
客慕coolvacuum 冻干机

